Overview
Endpoint’s pathology services deliver evaluation, oversight, and consultative support across the full scope of preclinical research. Our pathologists contribute not only essential readouts but also critical guidance during study planning, where early input improves endpoint selection, tissue collection, and specimen processing. We also provide independent peer review for regulatory submissions, ensuring accuracy, clarity, and confidence in study outcomes.
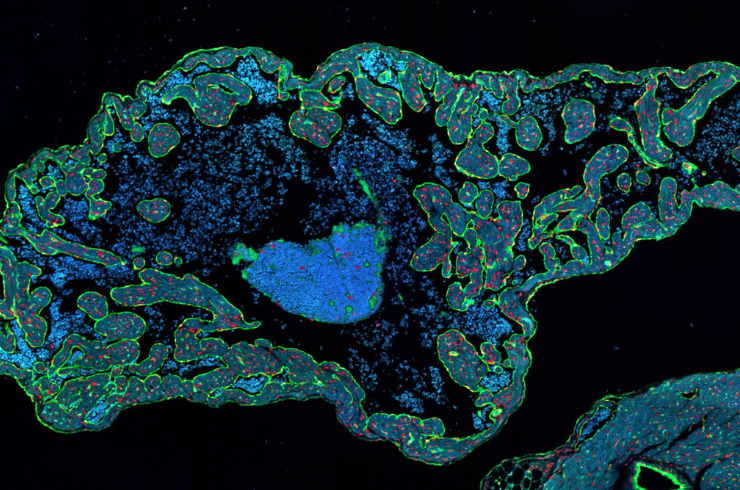
Our expertise spans anatomic and digital pathology evaluations, image-based biomarker analysis, and necropsy oversight across efficacy, safety, biodistribution, device–tissue interaction, and neurotoxicity studies. We also support analysis of novel models and non-traditional endpoints. These services apply to a wide range of biomedical products—including biomolecules, cell and gene therapies, medical devices, small molecules, and stem cells—helping programs align with translational goals and regulatory expectations.
Service Highlights
Below is a selection of our pathology offerings. If you don’t see exactly what you need, please contact us — we can likely tailor a solution for your program.
Why Endpoint?
Pathology at Endpoint is closely connected to other services, ensuring that findings are not assessed in isolation but are actively used to optimize study design and interpretation. By integrating pathology with surgical, model development, and toxicology expertise, Endpoint helps reduce rework, improve reproducibility, and deliver cohesive datasets. This collaborative model ensures that pathology insights contribute directly to advancing both therapeutic and device study objectives.