Overview
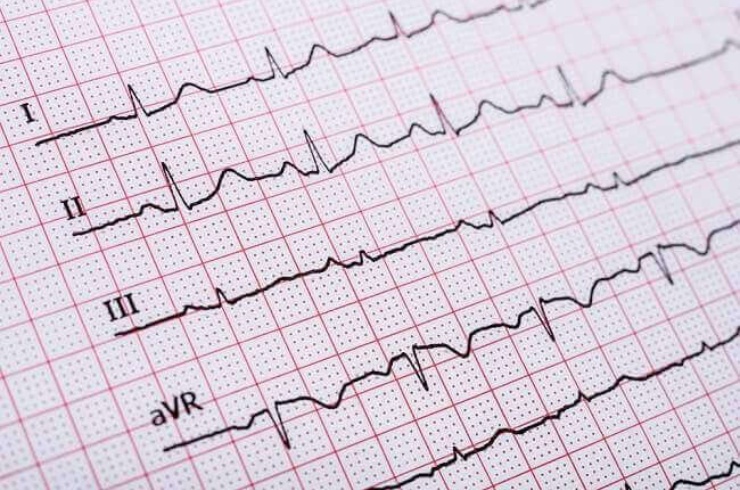
Endpoint Preclinical’s ECG & Respiratory Analysis service provides precise and reliable evaluation of cardiac electrical activity and respiratory function tailored to your preclinical research needs. We specialize in both qualitative and quantitative analyses—including arrhythmia detection, heart rate variability measurement, and rhythm interpretation, as well as detailed respiratory function evaluation. Our services support GLP and non‑GLP studies in safety pharmacology, efficacy assessments, toxicology evaluations, and academic research—ensuring that every relevant cardiorespiratory endpoint is captured with accuracy, efficiency, and rapid turnaround.
Service Highlights
Below is a selection of key ECG & Respiratory Analysis services that we offer. If you don’t see what you’re looking for, please contact us—we can likely help!
Why Endpoint?
Endpoint Preclinical provides comprehensive cardiorespiratory analytics, from raw telemetry signal processing to sophisticated statistical integration. By embedding ourselves within your existing workflow, we rapidly turn around high-volume data without compromising quality. Our team’s specialized knowledge of GLP-compliant reporting and advanced data visualization helps you capture nuanced endpoints—guiding crucial decisions in safety pharmacology, toxicology, and beyond.